
KardiaMobile Review: Is This Personal Heart Monitor Worth It?

Table of Contents
Introduction
KardiaMobile is a portable electrocardiogram (ECG) device designed for personal use, allowing individuals to monitor their heart rhythm without requiring a visit to a healthcare provider. Developed by AliveCor, this FDA-cleared device has emerged as the market leader in personal ECG technology, with over 200,000 units sold globally and adoption by major healthcare systems across the United States and Europe. The device has gained substantial popularity among those with heart health concerns, particularly individuals with arrhythmias such as atrial fibrillation (AFib), becoming one of the most physician-recommended personal heart monitoring solutions.
This widespread adoption is reflected in its consistent 4+ star ratings across major retail platforms and its frequent mention in cardiology journals and consumer health technology reviews. This review examines the KardiaMobile’s specifications, design, ease of use, performance, regulatory status, and overall value to help determine if this increasingly mainstream device is worth the investment for monitoring heart health.
Compare KardiaMobile Devices

| Feature | KardiaMobile (Standard) | KardiaMobile 6L | KardiaMobile Card |
|---|---|---|---|
| Price (when published) | $79 | $129 | $99 |
| Form Factor | Rectangular device (8.2 × 3.2 × 0.35 cm) | Rectangular device with fold-out electrode (8.2 × 3.2 × 0.35 cm) | Credit card-sized (8.6 × 5.4 × 0.18 cm) |
| Weight | 18g | 22g | 6g |
| Number of Leads | Single-lead ECG (Lead I) | Six-lead ECG (Leads I, II, III, aVL, aVR, aVF) | Single-lead ECG (Lead I) |
| Battery | Replaceable CR2016 coin cell | Replaceable CR2025 coin cell | Non-replaceable, 2-year battery life |
| Battery Life | ~12 months with typical use | ~12 months with typical use | ~2 years (not replaceable) |
| Connection Method | Ultrasonic transmission to smartphone | Ultrasonic transmission to smartphone | Ultrasonic transmission to smartphone |
| Water Resistance | No | No | Yes (IPX4 rated) |
| Recording Duration | 30 seconds (up to 5 min with Premium) | 30 seconds (up to 5 min with Premium) | 30 seconds (up to 5 min with Premium) |
| Detectable Conditions | Normal Sinus Rhythm, AFib, Bradycardia, Tachycardia | Normal Sinus Rhythm, AFib, Bradycardia, Tachycardia, plus enhanced detection of QT interval | Normal Sinus Rhythm, AFib, Bradycardia, Tachycardia |
| FDA Clearance | Yes | Yes | Yes |
| Method of Use | Place fingers on electrodes | Place fingers on top electrodes, bottom electrode against left leg for 6-lead | Place fingers on electrodes |
| Portability | Pocket-sized | Pocket-sized | Wallet-sized |
| Smartphone Attachment Option | Yes (adhesive plate included) | Yes (adhesive plate included) | No |
| Compatible with Kardia Premium | Yes | Yes | Yes |
| Suitable for | General users needing basic heart rhythm monitoring | Users needing more detailed ECG data or who have been recommended by physician for enhanced monitoring | Users prioritizing ultra-portability and durability |
Design and Build
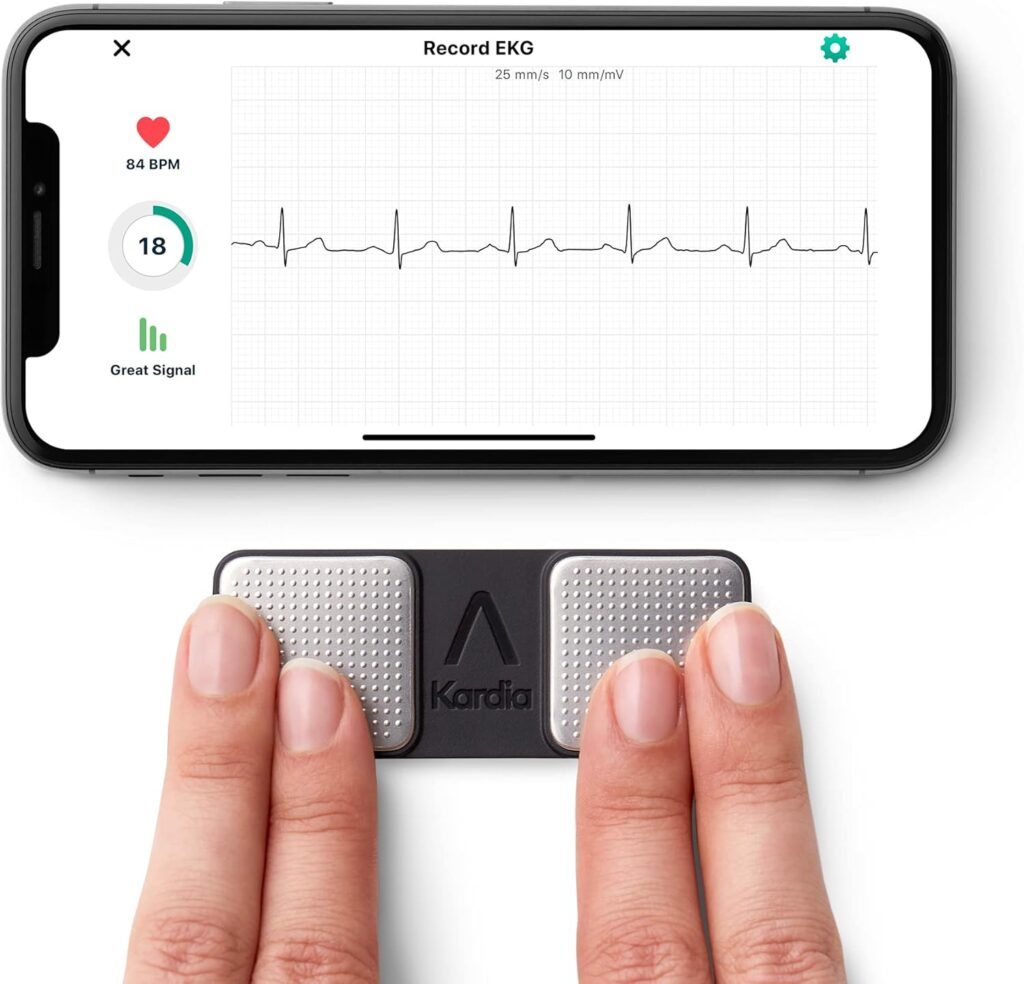
The KardiaMobile features a minimalist, clinical design that prioritizes function over aesthetics. The rectangular device houses two metal electrode pads on its surface, which users place their fingers on to capture the electrical activity of the heart. The construction utilizes medical-grade materials that feel substantial despite the device’s lightweight nature, inspiring confidence in its durability for daily use. The absence of any screens or complicated controls on the device itself contributes to its intuitive nature and streamlined appearance, reflecting AliveCor’s strategic decision to leverage the power of smartphones rather than incorporating expensive display technology into the device itself.

The metal electrodes are precisely aligned and firmly embedded in the plastic housing, showing no signs of loosening even after repeated use. The smooth, sealed construction makes the device easy to clean with an alcohol wipe, an important consideration for a product that will be handled frequently. Additionally, the compact form factor was clearly designed with portability in mind, allowing the device to slip easily into a pocket or attach to the back of a smartphone with the included adhesive plate, creating an integrated unit that encourages regular use by piggybacking on the smartphone that most users already carry.
While the KardiaMobile lacks the premium materials found in some consumer electronics, its medical-focused design appropriately emphasizes reliability over luxury. The single-piece construction eliminates seams or joints that could potentially collect dust or become failure points after extended use. The device’s ingenious design leverages the computational power and connectivity of smartphones, transmitting the ECG signal through an ultrasonic audio connection that works across various smartphone models without requiring Bluetooth pairing or additional hardware. This smartphone integration transforms the simple electrode device into a sophisticated medical monitoring system, with the Kardia app handling the complex signal processing, analysis, storage, and sharing functions while keeping the hardware component affordable and uncomplicated.
Setup and Use
Setting up the KardiaMobile involves downloading the Kardia app from the App Store or Google Play Store. Users create an account with basic personal information. The app guides users through a straightforward pairing process. As previously mentioned, this uses audio signals to connect the ECG device to the smartphone, eliminating the need for complicated Bluetooth pairing procedures.
The single-lead ECG produced by the standard KardiaMobile provides surprisingly detailed information. This is despite its simplified approach compared to the standard 12-lead ECG used in clinical settings. The device captures Lead I of the traditional ECG configuration. This measures the electrical potential difference between the left and right arms, sufficient for detecting many common arrhythmias, particularly atrial fibrillation.
Using the KardiaMobile on a daily basis is remarkably straightforward. It requires only 30 seconds of contact with the electrodes while remaining relatively still. The app provides real-time feedback during the recording process. It displays the ECG waveform as it’s being captured and offers guidance if signal quality is compromised due to excessive movement or improper finger placement.
The KardiaMobile system offers several methods for sharing results with medical professionals, enhancing its utility as a monitoring tool. Users can email ECG recordings directly from the app, print physical copies, or grant their physicians direct access to their ECG history through the Kardia Pro platform if their healthcare provider subscribes to this service. The app maintains a chronological history of all recordings, allowing users to track changes in their heart rhythm over time and identify patterns that might be relevant to their cardiac health. Additionally, the premium subscription unlocks advanced features like enhanced filtering for clearer readings, longer recording durations, and more detailed monthly heart health reports that provide greater insight into cardiac trends.
Premium subscription features offer enhanced filtering capabilities that can sometimes salvage recordings affected by minor interference. However, severe disruptions will still necessitate repeating the measurement under improved conditions.
The app effectively guides users to optimize their technique. However, factors such as dry skin, tremors, or nearby electronic interference can degrade signal quality. This occasionally results in “unclassified” readings that require manual review or repetition.
Quality of ECG Results
The single-lead ECG produced by the standard KardiaMobile provides surprisingly detailed information despite its simplified approach compared to the standard 12-lead ECG used in clinical settings. The device captures Lead I of the traditional ECG configuration, which measures the electrical potential difference between the left and right arms, sufficient for detecting many common arrhythmias, particularly atrial fibrillation. Independent studies have validated the accuracy of KardiaMobile’s atrial fibrillation detection algorithm, with sensitivity and specificity rates consistently above 90% when compared to physician-interpreted ECGs, demonstrating clinical relevance despite its consumer-oriented design.
The KardiaMobile 6L represents a significant advancement by providing six leads (I, II, III, aVL, aVR, and aVF) rather than just Lead I. This expanded lead configuration is achieved through an innovative design featuring two electrodes on the top surface for the thumbs and a third electrode on the bottom that contacts the user’s left knee or ankle. By capturing the heart’s electrical activity from multiple angles, the 6L provides substantially more diagnostic data while maintaining the platform’s accessibility. The enhanced lead configuration offers greater spatial resolution of cardiac electrical activity, potentially revealing abnormalities that might be missed on a single-lead recording, including axis deviation, ventricular hypertrophy, and bundle branch blocks.
The quality of the ECG trace depends significantly on proper technique and environmental conditions. Recordings taken while the user is seated calmly with hands resting on a flat surface typically yield the clearest results with minimal interference.
For users with intermittent symptoms, both KardiaMobile models offer a significant advantage over traditional monitoring methods due to their immediacy and accessibility. Unlike Holter monitors that require professional application and removal, or event recorders that may miss the onset of an episode, the KardiaMobile can be activated within seconds when symptoms occur, increasing the likelihood of capturing relevant cardiac events. This capability proves especially valuable for conditions like paroxysmal atrial fibrillation that may occur unpredictably and resolve before a patient can reach a medical facility, giving physicians documentary evidence of transient arrhythmias that might otherwise go unrecorded.
The 6L model provides particular value for patients with specific cardiac conditions beyond arrhythmias, as it enables more accurate QT interval measurement, which is critical for monitoring patients on medications that can affect cardiac repolarization. Many cardiologists find the 6L recordings significantly more useful for remote patient monitoring, as the additional leads provide context that helps distinguish between benign anomalies and potentially serious electrical disturbances requiring intervention. For patients with complex cardiac histories, the additional diagnostic value typically outweighs the minor inconvenience of the more complex recording position.

Even with these impressive advances, both KardiaMobile models have limitations. The standard model’s single-lead design cannot provide the comprehensive cardiac view afforded by a 12-lead ECG. While excellent for rhythm analysis, it cannot reliably detect conditions like myocardial ischemia or infarction that require multiple leads to visualize. The 6L improves upon this but still captures only half of the standard clinical leads. Additionally, the reliance on finger contact means that recordings cannot be taken during physical activity when motion artifacts would render the readings uninterpretable, limiting its utility during exercise-induced symptoms. The 6L’s three-point contact can sometimes provide more stable readings, but still requires the user to remain relatively still during recording.
Despite these constraints, both KardiaMobile models deliver impressive diagnostic value within their intended scope, providing clinically actionable information that far exceeds what was previously available in the consumer health device market. The choice between the standard model and the 6L ($79 versus $129) largely depends on the user’s specific health needs, with the 6L offering more comprehensive monitoring for those with complex cardiac conditions or medication regimens that affect cardiac electrical activity.
KardiaMobile FDA Clearance vs Full FDA Approval
The KardiaMobile has received FDA clearance rather than FDA approval, a distinction that reflects the regulatory pathway through which the device reached the market. FDA clearance is granted through the 510(k) premarket notification process, which allows devices to be marketed if they are shown to be “substantially equivalent” to legally marketed predicate devices that don’t require premarket approval. This process is less rigorous than the premarket approval (PMA) pathway required for Class III medical devices, which typically involves clinical trials demonstrating safety and efficacy. The clearance status indicates that the FDA has determined that the KardiaMobile is at least as safe and effective as similar previously cleared devices rather than requiring independent proof of efficacy.
The specific clearances for KardiaMobile cover its use as a single-channel ECG recorder and its algorithms for detecting atrial fibrillation, bradycardia, tachycardia, and normal sinus rhythm. These clearances allow AliveCor to market the device for these specific intended uses, but the company must be careful not to make claims beyond these cleared indications. The regulatory status means that while the device has been evaluated by the FDA and determined to meet applicable requirements for its intended use, it hasn’t undergone the more extensive review process required for novel, high-risk medical devices. This distinction is important for users to understand when evaluating the regulatory validation behind the device’s functionality and its clinical applications.
The practical implication of FDA clearance versus approval primarily affects how healthcare providers integrate the device into clinical practice. Many insurance companies and healthcare systems have established protocols regarding the use of FDA-cleared versus FDA-approved devices in treatment decisions, potentially influencing reimbursement policies and the weight given to KardiaMobile readings in clinical decision-making. Despite these considerations, the KardiaMobile’s FDA clearance provides substantial regulatory validation compared to non-cleared consumer health products, and many cardiologists have embraced the technology as a valuable screening and monitoring tool. Several peer-reviewed studies published in respected medical journals have further validated the device’s accuracy beyond its regulatory clearance, providing additional confidence in its clinical utility independent of its regulatory classification.
Value
With a base price of approximately $79 for the standard model and $129 for the six-lead KardiaMobile 6L (at publication), the KardiaMobile represents a significant investment compared to basic health monitoring devices like blood pressure monitors or pulse oximeters. This initial cost is supplemented by the optional Kardia Premium subscription, priced around $99 annually, which unlocks enhanced features like unlimited storage, detailed monthly reports, and the ability to share data directly with healthcare providers. When evaluating the value proposition, users must consider both the upfront hardware cost and the potential ongoing subscription expense against the device’s clinical benefits and potential reduction in conventional healthcare expenses.
For individuals with known or suspected cardiac arrhythmias, particularly those experiencing intermittent symptoms, the KardiaMobile can potentially reduce healthcare costs by decreasing the need for emergency room visits or extended monitoring periods. The ability to obtain immediate feedback during symptomatic episodes provides peace of mind that may prevent unnecessary medical visits while also capturing documentation that can expedite diagnosis when symptoms do represent significant arrhythmias. Additionally, the device may reduce the time to diagnosis for conditions like paroxysmal atrial fibrillation, which can be difficult to capture with traditional monitoring methods, potentially leading to earlier intervention and prevention of complications like stroke.
When compared to traditional cardiac monitoring options, the KardiaMobile offers exceptional value despite its cost. Professional Holter monitoring typically costs $100-$400 per 24-48 hour monitoring period, while event monitors may cost $250-$500 for a 30-day period, both requiring prescription and professional application. Against these alternatives, the KardiaMobile provides unlimited monitoring opportunities over several years for a comparable or lower price, though with the tradeoff of requiring active user participation rather than continuous passive recording. For patients with infrequent symptoms or those who need ongoing monitoring after an initial diagnosis, the long-term cost-effectiveness of the KardiaMobile becomes even more apparent, especially considering the device’s battery can last approximately one year before requiring replacement.
$99.00
| $79.00
| |
Compact
Most Popular
When you purchase through links on our site, we may earn an affiliate commission at no cost to you.
Conclusion
The KardiaMobile successfully bridges the gap between consumer health gadgets and medical-grade diagnostic equipment, offering genuinely useful clinical information in an accessible format. Its ability to detect atrial fibrillation with clinical-grade accuracy represents a significant advancement in preventive cardiology, potentially identifying this major stroke risk factor before catastrophic outcomes occur. The device’s straightforward operation makes it accessible to users of all technical abilities, while its integration with smartphones leverages existing technology to create a powerful health monitoring system without requiring additional screens or complex interfaces.
Despite its impressive capabilities, potential buyers should maintain realistic expectations about the KardiaMobile’s limitations. The single-lead nature of the standard model restricts its diagnostic scope, and the device cannot replace comprehensive cardiology evaluation for concerning symptoms. Additionally, the optional premium subscription model means that users must either accept limited functionality or commit to ongoing costs to access the full feature set. These considerations do not diminish the device’s core value but should factor into the purchasing decision, particularly for those considering it primarily for peace of mind rather than monitoring known conditions.
For individuals with diagnosed cardiac arrhythmias, unexplained palpitations, or risk factors for atrial fibrillation, the KardiaMobile offers exceptional value. It serves both as a monitoring tool and a means of communication with healthcare providers. The ability to capture symptomatic episodes when they occur and share objective data with physicians can significantly improve the diagnostic process and treatment plan development.
Healthcare technology increasingly emphasizes patient engagement and home monitoring, and the KardiaMobile exemplifies the positive potential of this trend when implemented thoughtfully. By combining clinical-grade analysis with consumer-friendly design, AliveCor has created a device that meaningfully advances cardiac care accessibility without sacrificing accuracy. While not without limitations and costs that require consideration, the KardiaMobile delivers on its core promise of bringing professional-quality ECG monitoring into the home environment, making it a worthwhile investment for those concerned about cardiac rhythm abnormalities who seek greater insight into their heart health between professional medical evaluations.
FAQ
The KardiaMobile has demonstrated high accuracy specifically for detecting atrial fibrillation, with studies showing sensitivity and specificity above 90% when compared to physician-interpreted 12-lead ECGs. However, it records only a single lead (comparable to Lead I in a traditional ECG) rather than the 12 leads used in clinical settings. This means it's excellent for rhythm analysis but cannot detect conditions that require multiple leads to visualize, such as myocardial ischemia or heart attacks. For its intended purpose of arrhythmia detection, particularly atrial fibrillation, the accuracy is sufficient for clinical use, though physicians typically confirm any significant findings with a full 12-lead ECG.
KardiaMobile does not recommend the use of Kardia products for patients with pacemakers, ICDs, or other implanted electronic devices. The accuracy could be affected.
No, the KardiaMobile cannot reliably detect heart attacks (myocardial infarction). Heart attack detection typically requires a 12-lead ECG that can visualize the electrical activity of the heart from multiple angles, as well as blood tests for cardiac enzymes. The single-lead KardiaMobile primarily detects rhythm abnormalities like atrial fibrillation, tachycardia, and bradycardia. While some heart attacks may cause rhythm changes that could appear on a KardiaMobile recording, many heart attacks would not show clear changes on a single-lead ECG. If you suspect you're having a heart attack, you should seek emergency medical attention immediately rather than relying on the KardiaMobile for diagnosis.
The KardiaMobile uses a 3V CR2016 coin cell battery that typically lasts approximately 12 months with normal usage patterns. When the battery power becomes low, the app will notify you that battery replacement is needed. The battery is user-replaceable, requiring a small screwdriver to open the battery compartment on the back of the device. Replacement batteries are inexpensive and widely available at pharmacies and electronics stores. The KardiaMobile 6L model uses a different battery (CR2025) but follows a similar replacement process. Proper battery replacement ensures the device will maintain optimal performance without requiring the purchase of a new unit.
The basic KardiaMobile functionality allows users to take unlimited 30-second ECG recordings, receive instant classifications (Normal, Possible Atrial Fibrillation, Bradycardia, Tachycardia, or Unclassified), and store a limited number of ECGs in the app. The premium subscription, which requires an additional annual fee, adds several enhanced features including unlimited cloud storage of ECG recordings, detailed monthly heart health reports, the ability to record ECGs up to 5 minutes in length, enhanced filters for clearer readings, and the ability to share data directly with healthcare providers through the Kardia Pro platform.
Premium subscribers also gain access to automatic clinical analysis of each ECG by a board-certified cardiologist (available only in certain regions). Whether the premium features justify the additional cost depends on individual monitoring needs and how frequently the device is used.
- The 2019 study published in the Journal of the American Heart Association that compared KardiaMobile to standard 12-lead ECGs for AFib detection in a primary care setting.
- Research published in Heart (BMJ Journals) which demonstrated high sensitivity and specificity for the KardiaMobile when compared to cardiologist-interpreted ECGs.
- The MOBILE-AF study (published in Europace) examining the effectiveness of KardiaMobile for AFib screening.
- Studies in Cardiovascular Digital Health Journal validating the accuracy of the six-lead KardiaMobile 6L device.
- Research in JAMA Cardiology examining the use of KardiaMobile in post-discharge monitoring of cardiac patients.
Review Scores
94%
Review Summary The KardiaMobile earns a strong 95% rating by successfully translating medical-grade ECG technology into an accessible, FDA-cleared consumer device with clinically validated accuracy for detecting arrhythmias, particularly atrial fibrillation. Its combination of portability, ease of use, and ability to provide immediate feedback during symptomatic episodes addresses a genuine need for between-appointment cardiac monitoring that traditional medical devices cannot match cost-effectively. While its single-lead design (or six-lead in the 6L model) limits some diagnostic capabilities compared to full 12-lead clinical ECGs, and the subscription model for premium features adds ongoing costs, the KardiaMobile delivers exceptional value for users seeking reliable heart rhythm monitoring in daily life.


























